Introduction: Pancreatic ductal adenocarcinoma (PDAC) is one of the most devastating cancers, and therapies to treat this malignancy have not evolved rapidly to fulfill the need of affected patients. PDAC, like many solid malignancies, is considered a “cold immunological tumor” where the lack of targetable tumor-associated antigens has hampered the development of successful immunotherapies. Despite newer treatment approaches, PDAC patients with advanced disease have a dismal prognosis. Therefore, there is an urgent need to develop new therapies. Plectin-1 is a high molecular weight protein (∼500 kDa) that participates in cytoskeleton network organization linking intermediate filaments to microtubules and microfilaments. Plectin-1 is aberrantly expressed in the PDAC cellular membrane, contributes to PDAC pathogenesis, and confers a poor prognosis. Plectin-1-targeting peptides (PTP) in mice and humans have been successfully explored for their use in PDAC imaging and targeted payload delivery (small molecules, radioisotopes, and chemotherapy). We sought to take advantage of the specific binding of PTP and develop a peptide-based plectin-1 chimeric antigen receptor (CAR), and test T-cell transduced with this construct in vitro against PDAC cell lines.
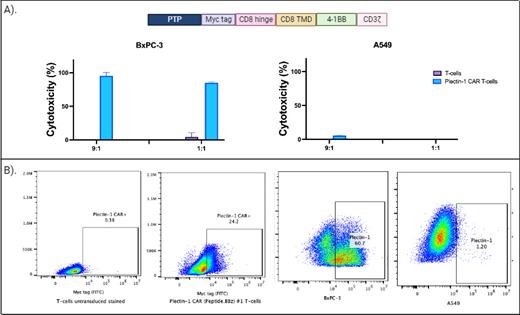
Methods: Using in silico tools, we optimized different pepto-CAR constructs and selected one that showed the highest levels of cytotoxicity against plectin-1-expressing PDAC cell lines. A myc tag was incorporated in the hinge region for flow cytometry or western blot detection. The intracellular signaling domain of these CAR sequences included 4-1BB and the zeta chain of the CD3 molecule ( Figure 1A). Plasmids containing the CAR sequence were packaged on lentivirus particles using HEK293T cells. Purified plectin-1 CAR lentiviruses were used to transduce T-cells derived from human peripheral blood T-cells obtained and purified from healthy donor whole blood. The expanded CAR T-cells were used for cytotoxic experiments against different cell lines.
Results: Expression of plectin-1 CAR was confirmed by flow cytometry using an anti-myc tag antibody ( Figure 1B). Plectin-1 expression was analyzed in different pancreatic cancer cell lines, and the BxPC-3 cells showed the highest expression level ( Figure 1B). Using a luciferase-based assay, we plated 50,000 BxPC-3 cells per well in duplicates with different effector-to-target (E:T) ratios. After 24 hours, luciferin was added, and the plate was read using a luminometer. As a negative control for effector cells, we use T-cells without a CAR ( Figure 1B), and for the target cells, we use the plectin-1 negative lung cancer cell line A549 ( Figure 1B). Plectin-1 CAR T-cells effectively killed BxPC-3 cells at different E:T ratios in a specific manner compared with controls using unmodified T-cells. Furthermore, no cytotoxicity was observed against plectin-1 negative A549 cells ( Figure 1A).
Conclusion: Our results show that a novel peptide-based CAR construct design against plectin-1 can induce specific cytotoxicity on plectin-1 expressing PDAC cell line BxPC-3. Furthermore, these data demonstrate the feasibility of using a CAR with a peptide-based antigen binding domain instead of the traditional antibody-derived scFv. This novel peptide-based CAR design offers alternatives for cellular therapy bioengineering. Moreover, our results can significantly impact immune-oncology therapeutic applications, particularly in challenging diseases like pancreatic cancer.
Figure 1 . A) In the above section, the Plectin-1 CAR construct is depicted. Cytotoxicity of unmodified T-cells and Plectin-1 CAR T-cells against BxPC-3 and A549 is shown below. B) Expression of Plectin-1 CAR in unmodified T-cells and Plectin-1 CAR T-cells is shown on the left. The expression of plectin-1 antigen on the surface of BxPC-3 cells and A549 cells is shown on the right. PTP: plectin-1 targeting peptide.
Disclosures
No relevant conflicts of interest to declare.